Find the latest information on COVID-19, including details for healthcare providers, individuals, and more.
Your Health
|
|
- COVID-19 Treatments and Therapeutics (HHS)
- COVID-19 Treatments (CDC)
- Evusheld is an investigational medicine that can help protect you from getting COVID-19. You may be eligible for Evusheld if you:
- Are moderately or severely immunocompromised and may not mount an adequate immune response to COVID-19 vaccination OR have a history of severe allergic reactions to COVID-19 vaccines, and
- Do not currently have COVID-19 and have not recently had close contact with someone with COVID-19, and
- Are an adult or adolescent ages 12 years and older weighing at least 88 pounds (40 kg).
- Know Your Treatment Options for COVID-19 (FDA)
The U.S. Food and Drug Administration issued an Emergency Use Authorization to allow the use of monoclonal antibodies for the treatment of mild to moderate symptoms of COVID-19 in adults and pediatric patients.
View the South Dakota Monoclonal Antibody Treatment flyer
What are monoclonal antibodies? Monoclonal antibodies are laboratory-made proteins that mimic the antibodies created by your immune system to fight off harmful viruses. Bamlanivimab + etesevimab and casirivimab + imdevimab are monoclonal antibodies that are specifically designed to protect against severe COVID-19 infection. The antibodies bind to the spike protein of the COVID-19 virus to stop the virus from entering your cells and continuing the infection.
- Who can get this treatment? Antibody treatment can be used by people with mild to moderate COVID-19 who:
- Test positive for SARS-CoV-2;
- Are within 10 days of the start of their symptoms;
- Are age 12 or older and weigh at least 88 pounds; and
- Are at high risk of progressing to severe COVID-19 infection or of needing to be admitted to a hospital because of COVID-19. Examples of chronic medical conditions include:
- Chronic kidney disease
- Diabetes
- Immunosuppressive disease
- Currently receiving immunosuppressive treatment
- Having a body mass index (BMI) greater than 25 (overweight or obese)
- Pregnancy
- Aged 65 years and older
- Cardiovascular disease (including congenital heart disease) or hypertension
- Chronic lung diseases (e.g., chronic obstructive pulmonary disease [COPD], asthma [moderate-to-severe], interstitial lung disease, cystic fibrosis, and pulmonary hypertension)
- Sickle cell disease
- Neurodevelopmental disorders (e.g., cerebral palsy) or other complex conditions (e.g., genetic or metabolic syndromes and severe congenital abnormalities)
- Having a medical-related technological dependence (e.g, tracheostomy, gastrostomy, or positive pressure ventilation [not related to COVID-19])
Individuals who meet high-risk criteria and test positive should contact their primary care physician about a referral for antibody treatment within three days of a positive test result and no later than 10 days after symptom onset.
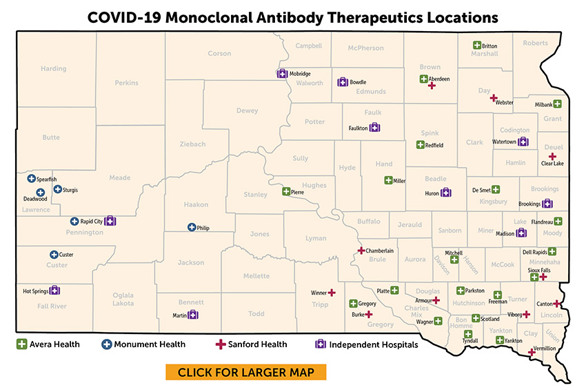
Treatment for COVID-19 is available in many parts of the state. Check with your healthcare provider about the use of monoclonal antibodies or an antiviral.
Avera Health
Monument Health
|
Sanford Health
Independent Hospitals
|
For Healthcare Providers
Healthcare Provider Guidance for COVID-19 (CDC)
Testing Recommendations
Medical providers are recommended to test individuals with signs and symptoms compatible with COVID-19 infection, including:
- Fever or chills
- Cough
- Shortness of breath or difficulty breathing
- Fatigue
- Muscle or body aches
- Headache
- Loss of taste or smell
- Sore throat
- Congestion or runny nose
- Nausea or vomiting
- Diarrhea
Care of Patients with COVID-19 Infection
When evaluating and providing medical care for a person with confirmed or suspected COVID-19 infection, please refer to CDC infection prevention and control recommendations.
- Long or Post-COVID Conditions (CDC)
- Clinical Care Quick Reference for COVID-19 (CDC)
- Cleaning, Disinfecting, and Ventilation (CDC)
Vaccine Guidance
- General Vaccine Guidance (CDC)
- Clinical & Professional Resources (CDC)
- Use of COVID-19 Vaccines in the United States (CDC)
- Vaccine Storage & Handling (CDC)
- Immunization Education & Training (CDC)
Resources
Guidance on specimen collection and testing, infection control practices, and other considerations are available at:
- Frequently Asked Questions to Assist Medicare Providers (CMS)
- Translated Resources and Materials for Medicare Providers (CMS)
- Clinician Outreach and Communication Activity (COCA) Calls/Webinars (CDC)
- Infection Control Guidance
- Clinical Care Guidance
- Special Clinical Considerations
- Interim Guidelines for Collecting and Handling of Clinical Specimens for COVID-19 Testing
SD Immunization Information System (SDIIS)
The SD DOH transitioned to a new immunization information system (IIS) vendor platform and is no longer creating user accounts in our legacy system. All users need to complete training to gain access to the new system. If your facility already uses SDIIS, please see this memo with instructions to complete training for our new system.
Need Assistance with SDIIS?
If you have questions or need assistance navigating the STC LMS, such as changing your job role, please contact STC staff at stc_trainingservices@stchome.com. If your facility is NOT using SDIIS, please get in touch with Brett Oakland at brett.oakland@state.sd.us to inquire about enrolling your facility in SDIIS.
Laboratory Resources
- South Dakota Public Health Lab website
- SARS-CoV-2 Laboratory Requisition and PUI Evaluation Form
- SARS-CoV-2 Sequencing Surveillance Laboratory Requisition Form
- Order Medical Shipping Supplies
- SD-DOH would like to remind medical providers, hospitals, and laboratories that cases of COVID-19, caused by the SARS-CoV-2 virus, are considered immediately reportable in South Dakota (under Coronavirus Respiratory syndromes).
Data Collection
Developing and using registries to collect, analyze, and share data about the COVID-19 virus and its impact on patients, physicians, and other caregivers will help develop the knowledge to successfully prevent and treat it. South Dakota clinicians and facilities have access to data collection tools to aid in the gathering of evidence. Learn more below:
Registry
The COVID-19 Positive Patient Registry is creating a database of all COVID-19-positive patients, regardless of their treatment. This registry collects data on onset symptoms, and pre-existing conditions but then also provides for the collection of treatments and outcomes initially and on an ongoing basis until the patient is recovered.
COVID-19 Positive Patient Registry
Medical Material Request & Fulfillment Policy
The SD DOH maintains a strategic stockpile of supplies readily available for emergency response. Requests received outside of a public health emergency must be evaluated by the state’s public health preparedness and response team.
Medical Material (PPE/Equipment) Request Fulfillment Policy
Test Kits Available
COVID-19 test kits are available through the South Dakota Public Health Laboratory. If you need kits, please use the online order form.