COVID-19 information and resources for the public on precautions to avoid illness, vaccines, symptoms, treatments, and therapeutics.
|
|
- COVID-19 Treatments and Therapeutics (HHS)
- COVID-19 Treatments (CDC)
- Evusheld is an investigational medicine that can help protect you from getting COVID-19. You may be eligible for Evusheld if you:
- Are moderately or severely immunocompromised and may not mount an adequate immune response to COVID-19 vaccination OR have a history of severe allergic reactions to COVID-19 vaccines, and
- Do not currently have COVID-19 and have not recently had close contact with someone with COVID-19, and
- Are an adult or adolescent ages 12 years and older weighing at least 88 pounds (40 kg).
- Know Your Treatment Options for COVID-19 (FDA)
The U.S. Food and Drug Administration issued an Emergency Use Authorization to allow the use of monoclonal antibodies for the treatment of mild to moderate symptoms of COVID-19 in adults and pediatric patients.
View the South Dakota Monoclonal Antibody Treatment flyer
What are monoclonal antibodies? Monoclonal antibodies are laboratory-made proteins that mimic the antibodies created by your immune system to fight off harmful viruses. Bamlanivimab + etesevimab and casirivimab + imdevimab are monoclonal antibodies that are specifically designed to protect against severe COVID-19 infection. The antibodies bind to the spike protein of the COVID-19 virus to stop the virus from entering your cells and continuing the infection.
- Who can get this treatment? Antibody treatment can be used by people with mild to moderate COVID-19 who:
- Test positive for SARS-CoV-2;
- Are within 10 days of the start of their symptoms;
- Are age 12 or older and weigh at least 88 pounds; and
- Are at high risk of progressing to severe COVID-19 infection or of needing to be admitted to a hospital because of COVID-19. Examples of chronic medical conditions include:
- Chronic kidney disease
- Diabetes
- Immunosuppressive disease
- Currently receiving immunosuppressive treatment
- Having a body mass index (BMI) greater than 25 (overweight or obese)
- Pregnancy
- Aged 65 years and older
- Cardiovascular disease (including congenital heart disease) or hypertension
- Chronic lung diseases (e.g., chronic obstructive pulmonary disease [COPD], asthma [moderate-to-severe], interstitial lung disease, cystic fibrosis, and pulmonary hypertension)
- Sickle cell disease
- Neurodevelopmental disorders (e.g., cerebral palsy) or other complex conditions (e.g., genetic or metabolic syndromes and severe congenital abnormalities)
- Having a medical-related technological dependence (e.g, tracheostomy, gastrostomy, or positive pressure ventilation [not related to COVID-19])
Individuals who meet high-risk criteria and test positive should contact their primary care physician about a referral for antibody treatment within three days of a positive test result and no later than 10 days after symptom onset.
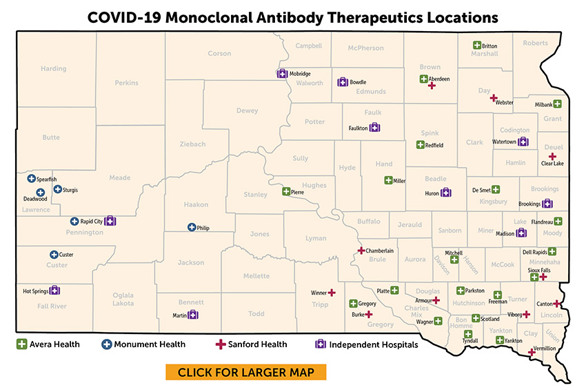
Treatment for COVID-19 is available in many parts of the state. Check with your healthcare provider about the use of monoclonal antibodies or an antiviral.
Avera Health
Monument Health
|
Sanford Health
Independent Hospitals
|